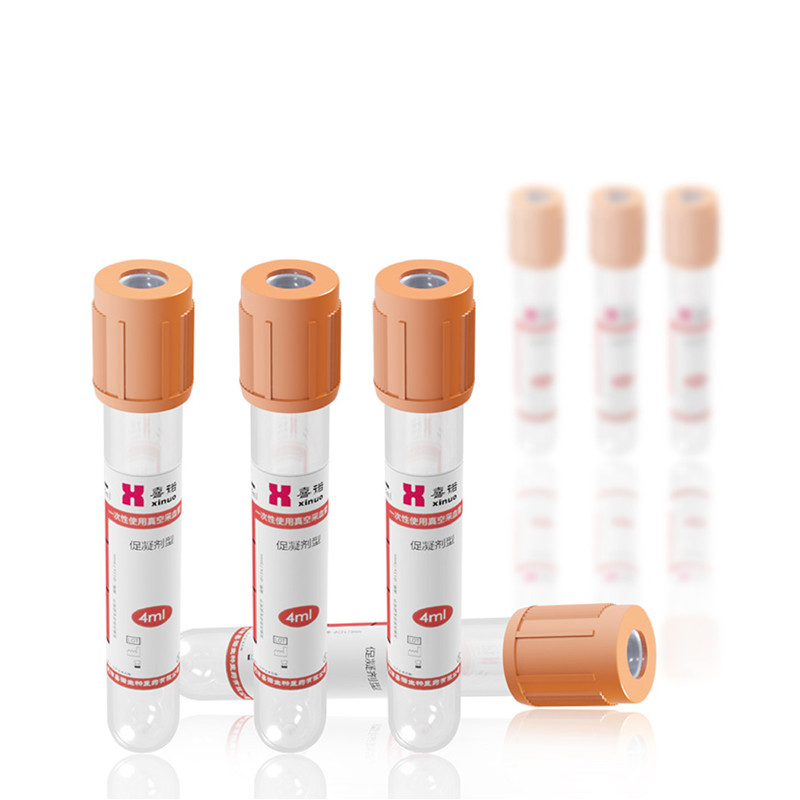
Vacuum Blood Collection Tube (Clot Activator) - Factory, Suppliers, Manufacturers from China
Vacuum Blood Collection Tube (Clot Activator), , , ,. The product will supply to all over the world, such as Europe, America, Australia,, ,, .
Related Products