Reasonable price for aspergillus galactomannan BAL - COVID-19 Antigen Lateral Flow Assay – Genobio
Reasonable price for aspergillus galactomannan BAL - COVID-19 Antigen Lateral Flow Assay – Genobio Detail:
Product Introduction
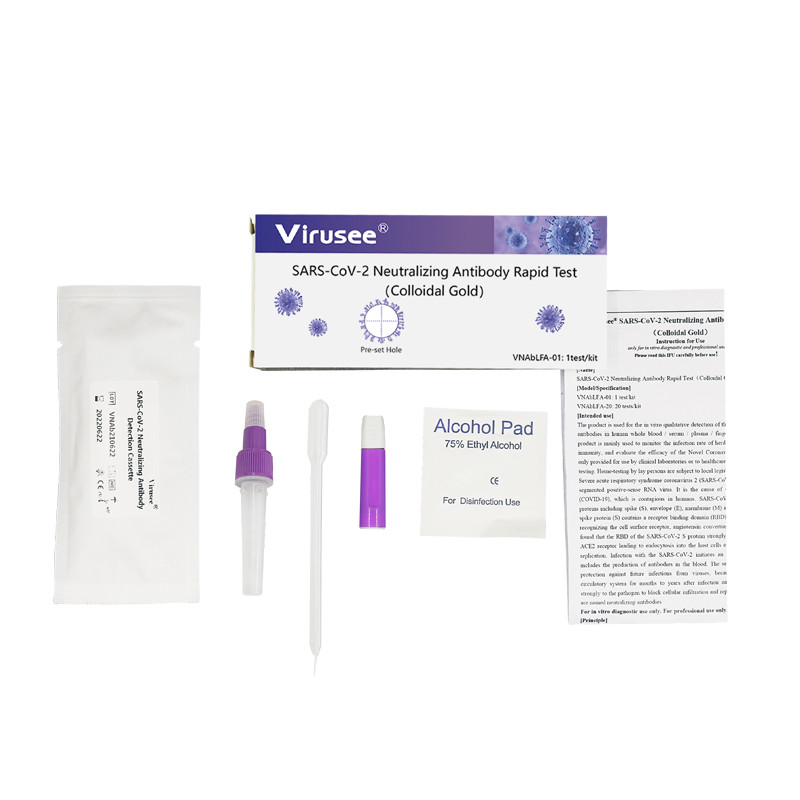
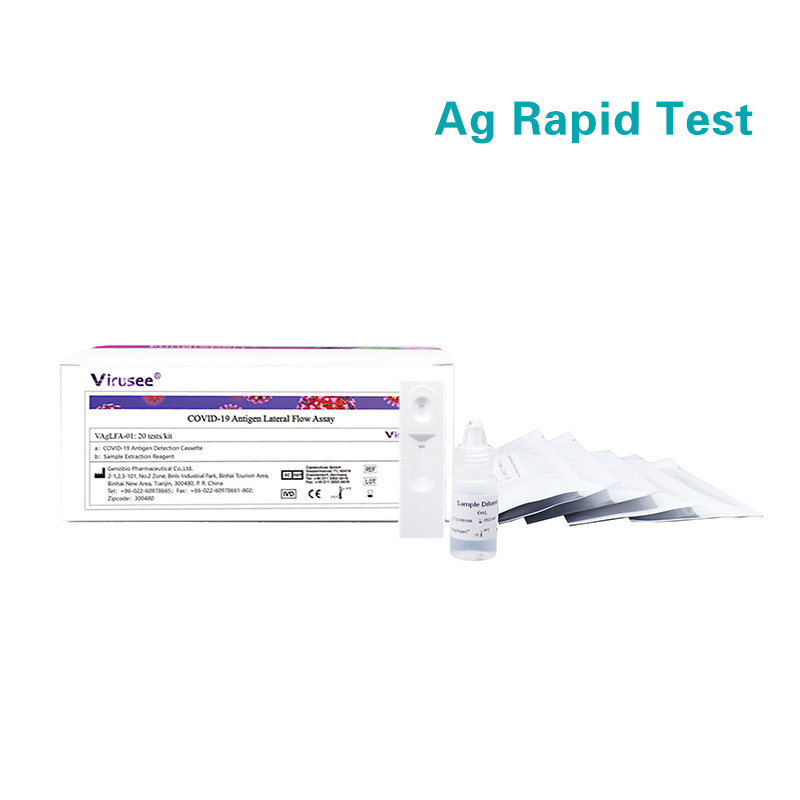
Virusee® COVID-19 Antigen Lateral Flow Assay is a lateral flow immunoassay intend for the qualitative detection SARS-CoV-2 nucleocapsid protein antigens in nasopharyngeal swab and oropharyngeal swab from individuals who are suspected of COVID-19 by their healthcare provider. Equipped with most consumables needed, it is rapid, accurate, cost-effective and user-friendly.
*Currently under evaluation of WHO Emergency Use Listing (EUL). (Application number EUL 0664-267-00).
Characteristics
|
Name |
COVID-19 Antigen Lateral Flow Assay |
|
Method |
Lateral Flow Assay |
|
Sample type |
Nasopharyngeal swab, Oropharyngeal swab |
|
Specification |
20 tests/kit |
|
Detection time |
15 min |
|
Detection objects |
COVID-19 |
|
Stability |
The kit is stable for 1 year at 2-30°C |

Advantage
- More choices, more flexibility
Samples applicable: Nasopharyngeal swab, oropharyngeal swab
For saliva test or single serving test kit – choose SARS-CoV-2 Antigen Rapid Test! - Rapid test, easy and fast
Obtain result within 15 min
Visually reading result, easy to interpret
Minimum manual operation, tools provided within the kit
- Convenient and cost-saving
Product can be transported and stored at room temperature, reducing costs - Included in China white list
- Currently under evaluation of WHO Emergency Use Listing (EUL). (Application number EUL 0664-267-00)
What is COVID-19?
In March 2020, the World Health Organization (WHO) declared the COVID-19 outbreak a pandemic. The virus is known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The disease it causes is called coronavirus disease 2019 (COVID-19).
Signs and symptoms of coronavirus disease 2019 (COVID-19) may appear 2 to 14 days after exposure. Common signs and symptoms can include: fever, cough, tiredness, or even a loss of taste or smell, breathing difficulty, muscle aches, chills, sore throat, runny nose, headache, chest pain, etc.
The virus that causes COVID-19 spreads easily among people. Data has shown that the COVID-19 virus spreads mainly from person to person among those in close contact (within about 6 feet, or 2 meters). The virus spreads by respiratory droplets released when someone with the virus coughs, sneezes, breathes, sings or talks. These droplets can be inhaled or land in the mouth, nose or eyes of a person nearby.
Globally, there have been over 258,830,000 confirmed cases of COVID-19, including 5,170,000 deaths reported. A rapid and accurate way for COVID-19 diagnosis is crucial for public healthcare and epidemic control.
Test process



Order Information
|
Model |
Description |
Product code |
|
VAgLFA-01 |
20 test/kit |
CoVAgLFA-01 |
Product detail pictures:







Related Product Guide:
Reasonable price for aspergillus galactomannan BAL - COVID-19 Antigen Lateral Flow Assay – Genobio , The product will supply to all over the world, such as: , , ,