Personlized Products OXA-23 - COVID-19 IgM Lateral Flow Assay – Genobio
Personlized Products OXA-23 - COVID-19 IgM Lateral Flow Assay – Genobio Detail:
Product Introduction
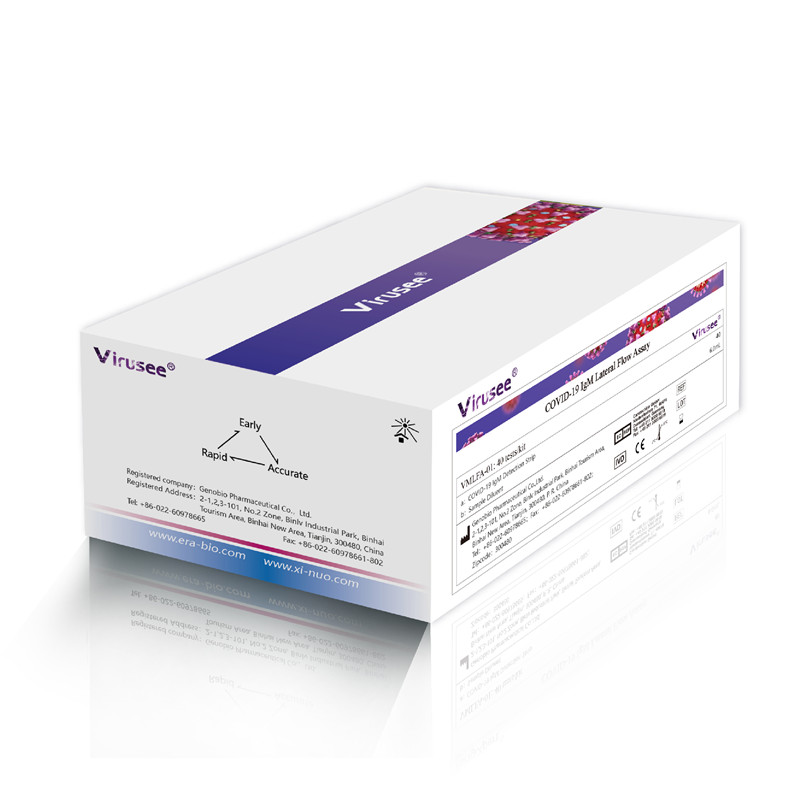
Virusee® COVID-19 IgM Lateral Flow Assay is a lateral flow immunoassay used for the qualitative detection of Novel Coronavirus IgM antibody in human whole blood / serum / plasma samples in vitro. It is mainly used in the auxiliary clinical diagnosis of novel coronavirus pneumonia.
The novel coronavirus is a positive single-stranded RNA virus. Unlike any known coronavirus, the vulnerable population for Novel Coronavirus is generally susceptible, and it is more threatening to the elderly or people with fundamental diseases. IgM antibodies positive is an important indicator of novel coronavirus infections. Detection of novel coronavirus-specific antibodies will aid clinical diagnosis.
Characteristics
|
Name |
COVID-19 IgM Lateral Flow Assay |
|
Method |
Lateral Flow Assay |
|
Sample type |
Blood, plasma, serum |
|
Specification |
40 tests/kit |
|
Detection time |
10 min |
|
Detection objects |
COVID-19 |
|
Stability |
The kit is stable for 1 year at 2-30°C |

Advantage
- Rapid
Obtain result within 10 min - Simple
Visually reading result, easy to interpret
Simple procedure, without complicated operation
- Cost-saving
Product can be transported and stored at room temperature, reducing costs - Low risk
Testing blood sample, reducing the risk of sampling process - Suitable for screening on-site, bedside, outpatient
Background and principle
SARS-CoV-2 emerged as a novel virus with no available treatment option and caused a serious disaster across the world. The disease caused by this virus, “COVID-19″, announced a global pandemic on March 11, 2020. Without any proper treatment and vaccine for COVID-19, people around the world are currently experiencing a worldwide emergency affecting all societies, and it has sent billions of people into lockdown. Around the world, desperate efforts are underway to curtail this pandemic while it has resulted in the collapsing of health systems and has triggered lasting geopolitical and economic changes.
Poor diagnosis of COVID-19 has also contributed to disease severity due to stress (in case of false positive) and disease spread (in case of a false negative). A lack of RT-PCR test sampling of lower tract respiratory specimens was the main reason for the misclassification of symptomatic patients as either having COVID-19 or not. A prompt diagnosis with serological testing shows SARS-CoV-2 IgG/IgM patterns in a better and understandable way of seroconversion.
The IgG/IgM assays to detect the length and origin of humoral responses against SARS-CoV-2 is very important, and these antibodies can be detected from a few days after the onset of diseases and may remain in the body even after years of infection. In the case of COVID-19, IgM and IgG response can be observed from the second week of the disease.
Serologic assays provide quick diagnostic by avoiding PCR false positive/false negative result as well as these provide antibody pattern for estimation of strength and duration of humoral immunity.
IgM and IgG antibody detection can identify suspected cases with negative nucleic acid tests. Compared with nucleic acid detection, IgM and IgG detection may provide a quick, simple, and accurate detection method for suspected COVID-19 cases.


Test process

Order Information
|
Model |
Description |
Product code |
|
VMLFA-01 |
40 test/kit, strip format |
CoVMLFA-01 |
Product detail pictures:


Related Product Guide:
Personlized Products OXA-23 - COVID-19 IgM Lateral Flow Assay – Genobio , The product will supply to all over the world, such as: , , ,