OEM Manufacturer candida IgM IgG - Fungus (1-3)-β-D-Glucan Test (Chromogenic Method) – Genobio
OEM Manufacturer candida IgM IgG - Fungus (1-3)-β-D-Glucan Test (Chromogenic Method) – Genobio Detail:
Product Introduction
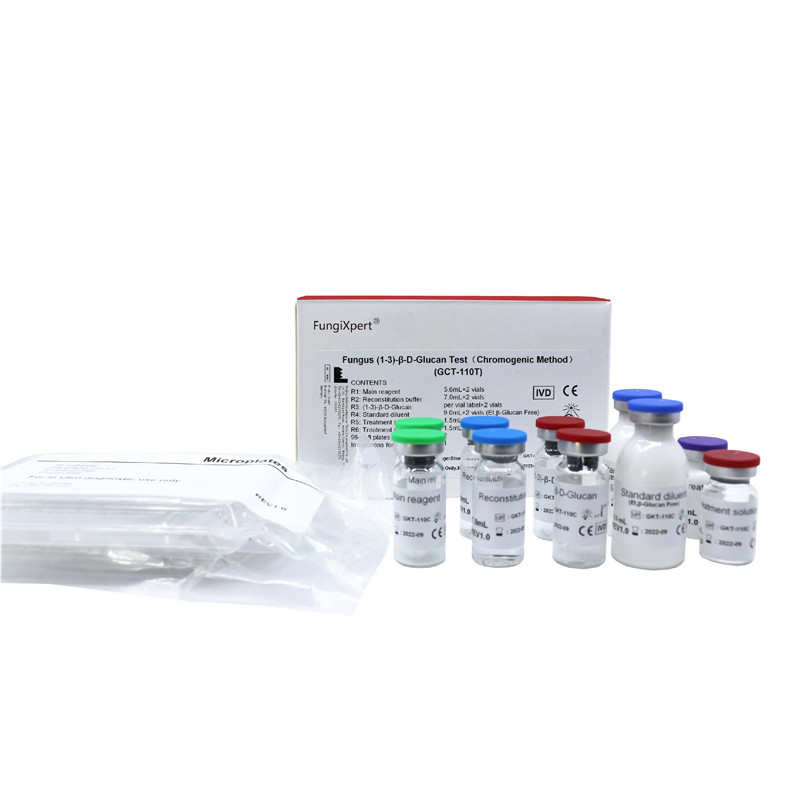
FungiXpert® Fungus (1-3)-β-D-Glucan Detection Kit (Chromogenic Method) is intended for the screening diagnosis of invasive fungal disease. It is used to provide rapid diagnostic reference for clinical invasive fungal infections through quantitative detection of (1-3)-β-D-glucan in the serum and Bronchoalveolar Lavage (BAL) fluid. GCT-110T series are intended for manual operation with microplate reader. GKT-5M/10M are used with our semi-automated instrument MB80 series and fully automated instrument IGL series.
Characteristics
|
Name |
Fungus (1-3)-β-D-Glucan Detection Kit (Chromogenic Method) |
||||
|
Model |
GCT-110T |
GKT-25M |
GKT-12M |
GKT-10M |
GKT-5M |
|
Specification |
110 tests/kit |
50 tests/kit |
50 tests/kit |
36 tests/kit |
30 tests/kit |
|
Detection time |
40 min |
60 min |
|||
|
Instrument |
Microplate Reader |
Kinetic Tube Reader |
|||
|
Method |
Chromogenic Method |
||||
|
Sample type |
Serum, BAL fluid |
||||
|
Detection objects |
Invasive fungi |
||||
|
Linearity range |
31.25-500 pg/mL |
||||
|
Stability |
Stable for 3 years at 2-8°C in the dark |
||||
Advantages
- Methodology advantages
Obtain results in 40-60 min, 5-8 days prior to clinical symptoms and imaging
Recommended by the EORTC/MSG Consensus Group as one of the mycological criteria for the diagnosis of invasive fungal disease - More options available:
Automated instruments / Manual operation
Larger / smaller throughput
Customization service - Quality controls are provided with the kit
More reliable experimental accuracy
- Compatible with Automatic instruments
Can be equipped with fully automatic instruments, easy and reduce errors
Fully Automatic Kinetic Tube Reader (IGL-200)
Fully Automatic Kinetic Tube Reader (IGL-800)
Kinetic Tube Reader (MB-80M)
Kinetic Tube Reader (MB-80A)
Kinetic Tube Reader (MB-80X) - Good manufacture quality and ability
Total manufacture from principal raw materials to finished products.
In 2017, Era Biology, as a leader and pioneer in the industry, participated in drafting the industry standard of “Fungus(1-3)-B-D-Glucan Test” with National Center for Clinical Laboratories etc.
Principle of BDG test
Fungus (1-3)-B-D-Glucan (BDG) is a unique element of fungal cell wall, when the fungus invades blood or deep tissue, BDG can be released from the cell wall.
Applicable departments
Order Information
|
Model |
Description |
Product code |
|
GCT-110T |
110 tests/kit, used with Microplate Reader |
BG110-001 |
|
GKT-12M |
50 tests/kit, used with automated Kinetic Tube Reader |
BG050-001 |
|
GKT-25M |
50 tests/kit, used with automated Kinetic Tube Reader |
BG050-002 |
|
GKT-5M |
30 tests/kit, used with automated Kinetic Tube Reader |
BG030-001 |
|
GKT-10M |
36 tests/kit, used with automated Kinetic Tube Reader |
BG030-002 |
Product detail pictures:


Related Product Guide:
OEM Manufacturer candida IgM IgG - Fungus (1-3)-β-D-Glucan Test (Chromogenic Method) – Genobio , The product will supply to all over the world, such as: , , ,