YY/T 1729-2020 "Fungus (1-3)-β-D-Glucan Test" formulated by Era Biology was approved by NMPA on July 9, 2020 and officially released. The standard will be formally implemented on June 1, 2021.
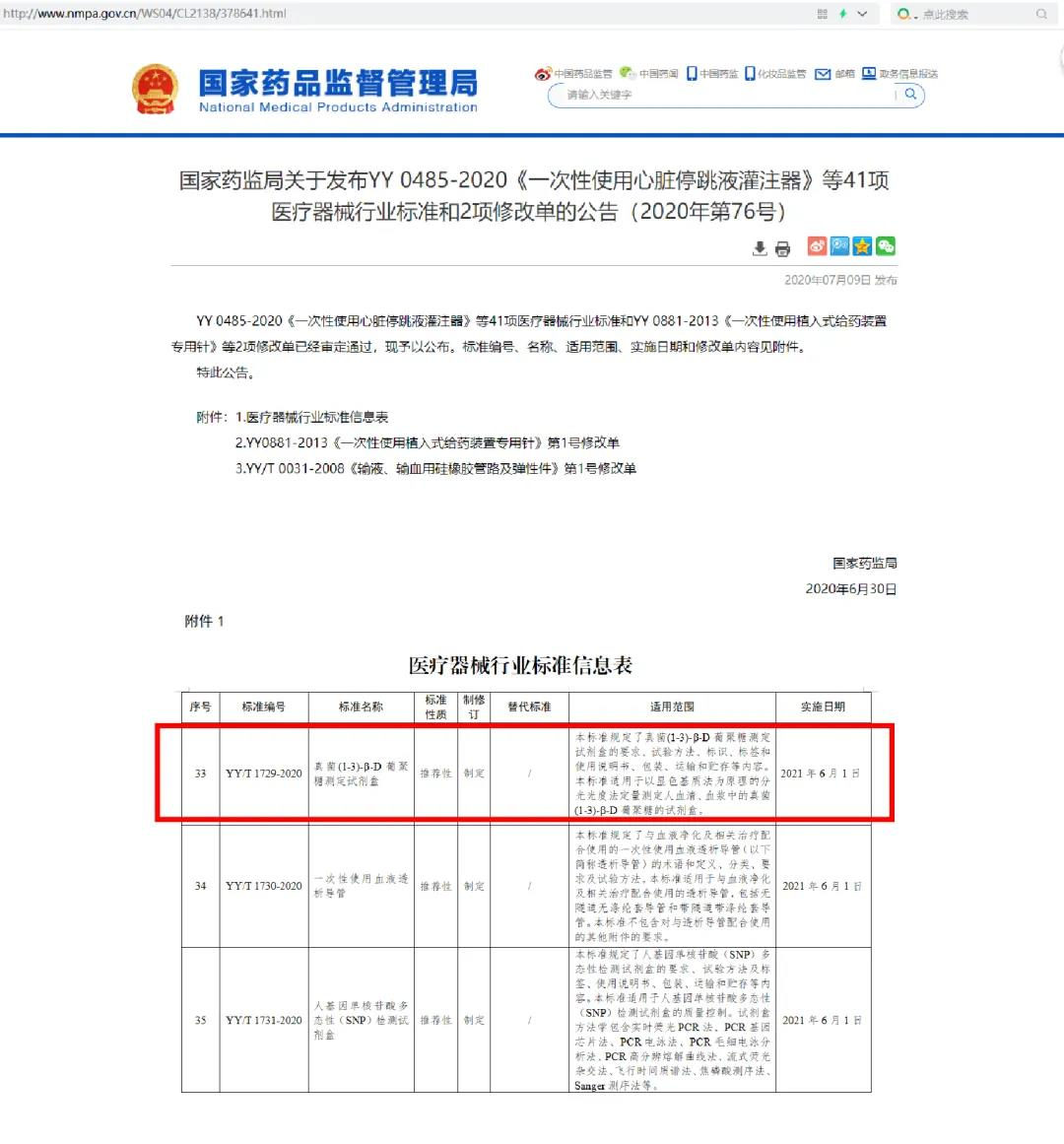
The preparation of this standard was organized by the National Medical Clinical Laboratory and the In Vitro Diagnostic System Standardization Technical Committee (TC136) , and it was officially launched in April 2017. Beijing Gold Mountainriver Tech Development Co., Ltd., a subsidiary of Era Biology, as the first drafter, cooperates with Beijing Medical Device Inspection Institute, Beijing Medical Device Technology Evaluation Center, National Health Commission Clinical Testing Center, and Genobio Pharmaceutical Co., Ltd. (a wholly-owned subsidiary of Era Biology), jointly drafted and formulated the standard. As the first industry standard in the field of fungus rapid detection, which is led by an enterprise, the standard stipulates the accuracy, linearity, blank limit, detection limit, and repeatability, bottle-to-batch difference, batch-to-batch difference, analysis specificity, stability requirements and test methods, etc., of the fungus (1-3)-β-D-glucan test. This standard is applicable to kits for the quantitative determination of fungal (1-3)-β-D glucan in human serum and plasma by spectrophotometry based on the principle of chromogenic method.
As a leading company in the domestic fungal rapid inspection industry, Era Biology not only fills the domestic gap in one fell swoop, but also developed the first rapid diagnostic product for invasive fungal diseases, and is also committed to the continuous upgrading of product standards. For more than 20 years, we have been positioned as an industry leader, guided by market standardization, constantly advancing with the times, striving for perfection, and continuing to pursue excellence. The formulation of this standard has shown the strength of the leading brand in the fungus testing to the industry. The promulgation of this standard can effectively standardize the quality of products in the industry and enhance the reputation of the fungal testing industry in the entire field of in vitro diagnostics.
Post time: Mar-31-2021
