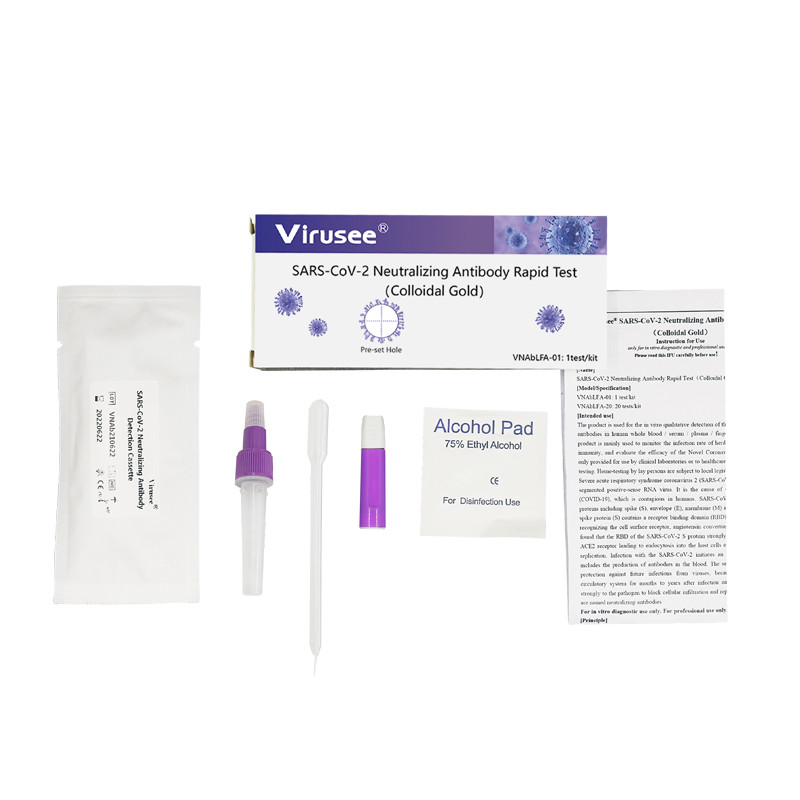
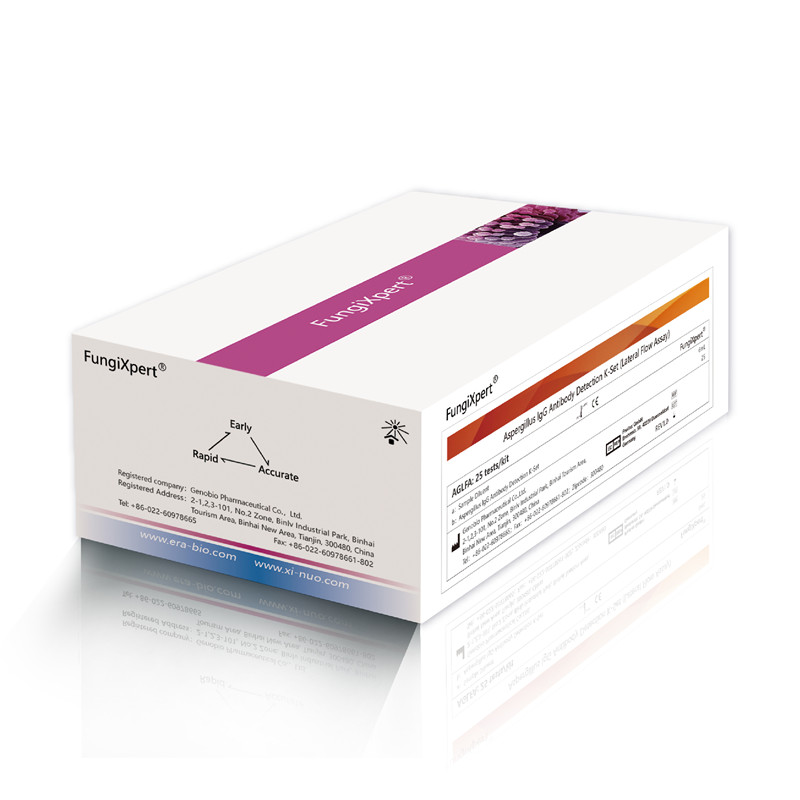
High Quality for candida IgG test - Aspergillus IgG Antibody Detection K-Set (Lateral Flow Assay) – Genobio
High Quality for candida IgG test - Aspergillus IgG Antibody Detection K-Set (Lateral Flow Assay) – Genobio Detail:
Product Introduction
FungiXpert® Aspergillus IgG Antibody Detection K-Set (Lateral Flow Assay) uses colloidal gold immunochromatography technology to detect aspergillus-specific IgG antibody in human serum, providing a rapid and effective auxiliary aid for the diagnosis of susceptible populations.
Invasive fungal diseases (IFD) have become one of the biggest life threats to immunocompromised patients and have caused high morality worldwide. Aspergillus species are ubiquitous, saprophytic fungi that are important causes of morbidity and mortality in transplant recipients. Humans become infected with Aspergillus after conidia are inhaled and deposited in bronchioles, in alveolar spaces, and less commonly in paranasal sinuses. Most common Aspergillus pathogens include Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, Aspergillus terreus.
Chronic pulmonary aspergillosis (CPA) is an underdiagnosed and misdiagnosed disease and now increasingly recognised. However, the diagnosis of CPA remains challenging. Recent studies have found the diagnostic values of serum Aspergillus-specific IgG and IgM antibodies in patients with CPA. The Infectious Diseases Society of America (IDSA) have recommended that Aspergillus IgG antibody elevated or other microbiological data is one of the necessary evidences for the diagnosis of chronic cavitary pulmonary aspergillosis (CCPA).
Characteristics
|
Name |
Aspergillus IgG Antibody Detection K-Set (Lateral Flow Assay) |
|
Method |
Lateral Flow Assay |
|
Sample type |
Serum |
|
Specification |
25 tests/kit; 50 tests/kit |
|
Detection time |
10 min |
|
Detection objects |
Aspergillus spp. |
|
Stability |
The K-Set is stable for 2 years at 2-30°C |
|
Low detection limit |
5 AU/mL |

Advantage
- Simple and accurate
Easy to use, ordinary laboratory staff can operate without training
Intuitive and visual reading result - Accurate and economical
Low detection limit: 5 AU/mL
Transported and stored at room temperature, reducing costs - Rapid and convenient
Obtain result within 10 min
Two specifications available: cassette/25T; strip/50T - Support the diagnosis of aspergillosis in early stage
Aspergillus-specific IgG antibodies take a mean of 10.8 days to appear in acute illness - Detection of single immunoglobulin subtype demonstrate the infection stage
Relationship between antibody concentration and Aspergillus infection

- Recommended by ESCMID/ECMM/ERS/IDSA etc
IgG antibody response to Aspergillus spp. is one of the characteristics required for the diagnosis of CPA.
Aspergillus IgG antibody elevated or other microbiological data is one of the necessary evidences for the diagnosis of chronic cavitary pulmonary aspergillosis (CCPA)
Antibody diagnosis of chronic pulmonary aspergillosis (CPA)
| Population | Intention | Intervention | SoR | QoE |
| Cavitary or nodular pulmonary infiltrate in non-immunocompromised patients |
Diagnosis or exclusion of CPA | Aspergillus IgG antibody | A | II |
- Applicable department
Respiration department
Cancer department
Hematology department
ICU
Transplantation department
Infectious department
Operation


Order Information
|
Model |
Description |
Product code |
|
AGLFA-01 |
25 tests/kit, cassette format |
FGM025-002 |
|
AGLFA-02 |
50 tests/kit, strip format |
FGM050-002 |
Product detail pictures:
Related Product Guide:
High Quality for candida IgG test - Aspergillus IgG Antibody Detection K-Set (Lateral Flow Assay) – Genobio , The product will supply to all over the world, such as: , , ,