Full-Automatic Chemiluminescence Immunoassay System (FACIS-I)
Product Introduction
Obtain quantitative, accurate result by chemiluminescence immunoassay with easiest operation and shortest time!
FACIS (Full-Automatic Chemiluminescence Immunoassay System) is a closed system using chemiluminescence immunoassay to acquire quantitative test results. It is for now capable to detect the content of (1-3)-β-D glucan, as well as the antigen and antibodies of Aspergillus spp., Candida spp., Cryptococcus app, 2019-nCOV, etc.
FACIS uses independent reagent cartridge design, fully automated operation steps, matching with intelligible and multi-functional software, to provide rapid and simple test process, and obtain accurate and quantitative results.
Characteristics
|
Name |
Full-Automatic Chemiluminescence Immunoassay System |
|
Model Analysis |
FACIS-I |
|
Analysis method |
Chemiluminescence immunoassay |
|
Detection time |
40 min |
|
Wavelength range |
450 nm |
|
Number of channels |
12 |
|
Size |
500mm×500mm×560mm |
|
Weight |
47 kg |

Advantages

Fully Automatic process
- Automatically proceed sample treatment, detection and analysis.
- 12 channels work simultaneously.
- Avoid mistakes in manual operation.
- Shorten the experimental time of multiple samples.
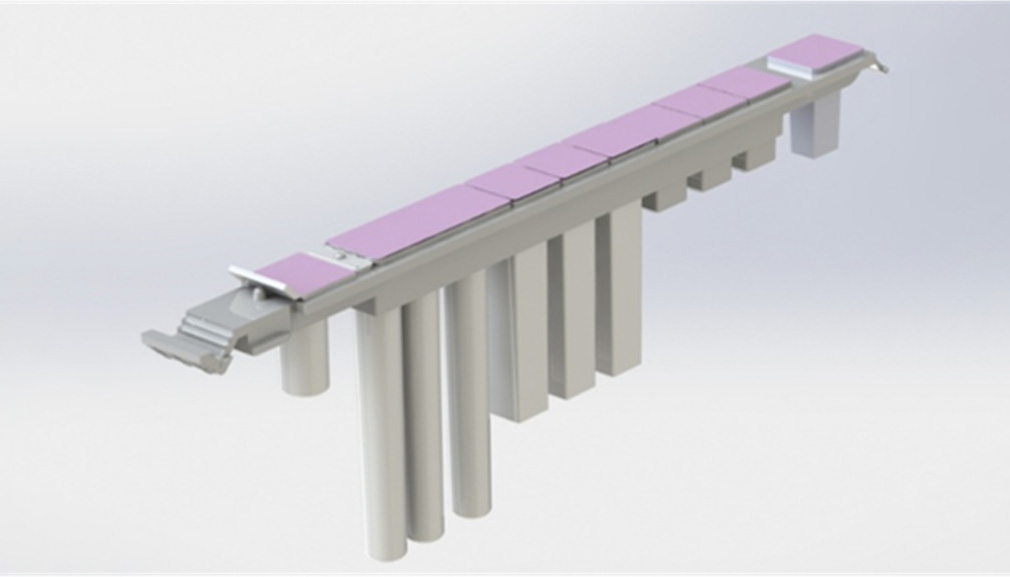
Independent reagent cartridge
- Uniform design specially for FACIS
- Infinite possibilities: More detection items in the future
- All in one: reagents, tips and processing positions in one strip. Convenient and avoids waste
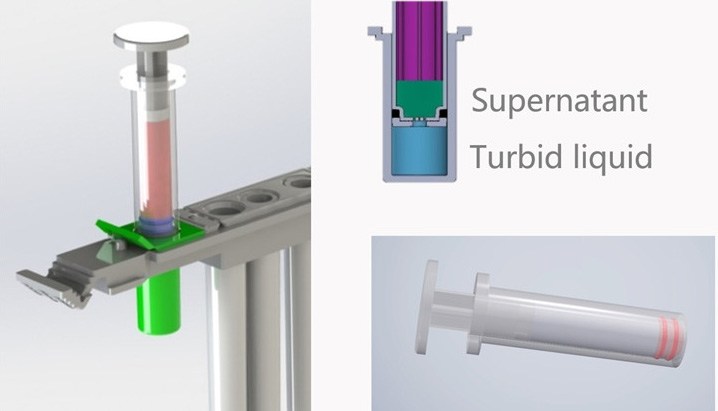
Special sample pretreatment system with invention patent
- Micron film is used to separate the treated sample
- Simplifies the operation procedure
- Sample separation method: Filtration
- Pre-treatment module: Metal Bath

Intelligent system
- Special software: shows steps of the operation, easy to operate
- Safety assurance: Automatic power cut-off protection and high-temperature warning
- Compact design: Saves lab space.
- Rapid: Total time of each run is only 60 min.
- Extensible: Multiple units can be used online, realizing LIS data share
Q&A
Q: How should we install the FACIS after receiving it?
A: The instruments sent to customers have already set all the parameters and done the calibration. No complicated installation is required. Just power on and try your first test according to the manual.
Q: How can I learn to use FACIS?
A: Operation of FACIS is very simple and convenient. Follow the manual and the indication of software. Also, we provide operation video and online training service to help you know better about FACIS.
Q: What preparation is required before performing the test?
A: In addition to general lab requirements, before performing tests on FACIS, reagents should be taken out from refrigerator and get to room temperature. Check whether the standard curve files of the batches you use have been imported into the system.
Q: What can FACIS test?
A: FACIS is compatible with all the CLIA (Chemiluminescence Immunoassay) reagent kits provided by our company, including antigen and antibody detection of Aspergillus, Cryptococcus, Candida, COVID-19 and so on. Due to its intelligent design and unique reagent cartridge, more and more reagents will be developed to be applicable to FACIS.
Q: How often should the quality controls be tested?
A: Positive controls and negative controls are provided within the CLIA reagent kits. It is recommended to perform controls each run, to better ensure the quality of test results.
Service
- Online training: Follow us to perform step by step.
- Trouble shooting: Professional engineer to help you figure out any problem.
- Update of new version of software & newly developed reagents.
Related videos
Order Information
Product code: FACIS-I










