China OEM Test Flu Influenza a&B Virus Antigen Diagnostic Test Kit
We’ve got quite possibly the most state-of-the-art production gear, experienced and qualified engineers and workers, acknowledged top quality handle systems along with a friendly expert gross sales group pre/after-sales support for China OEM Test Flu Influenza a&B Virus Antigen Diagnostic Test Kit, We’ve been extremely aware of excellent, and have the certification ISO/TS16949:2009. We are dedicated to supply you high quality solutions with acceptable price tag.
We’ve got quite possibly the most state-of-the-art production gear, experienced and qualified engineers and workers, acknowledged top quality handle systems along with a friendly expert gross sales group pre/after-sales support for China Influenza a&B Rapid Test and Flu Test Kit, Now we have a strict and complete quality control system, which ensures that each product can meet quality requirements of customers. Besides, all of our goods have been strictly inspected before shipment.
Product Introduction
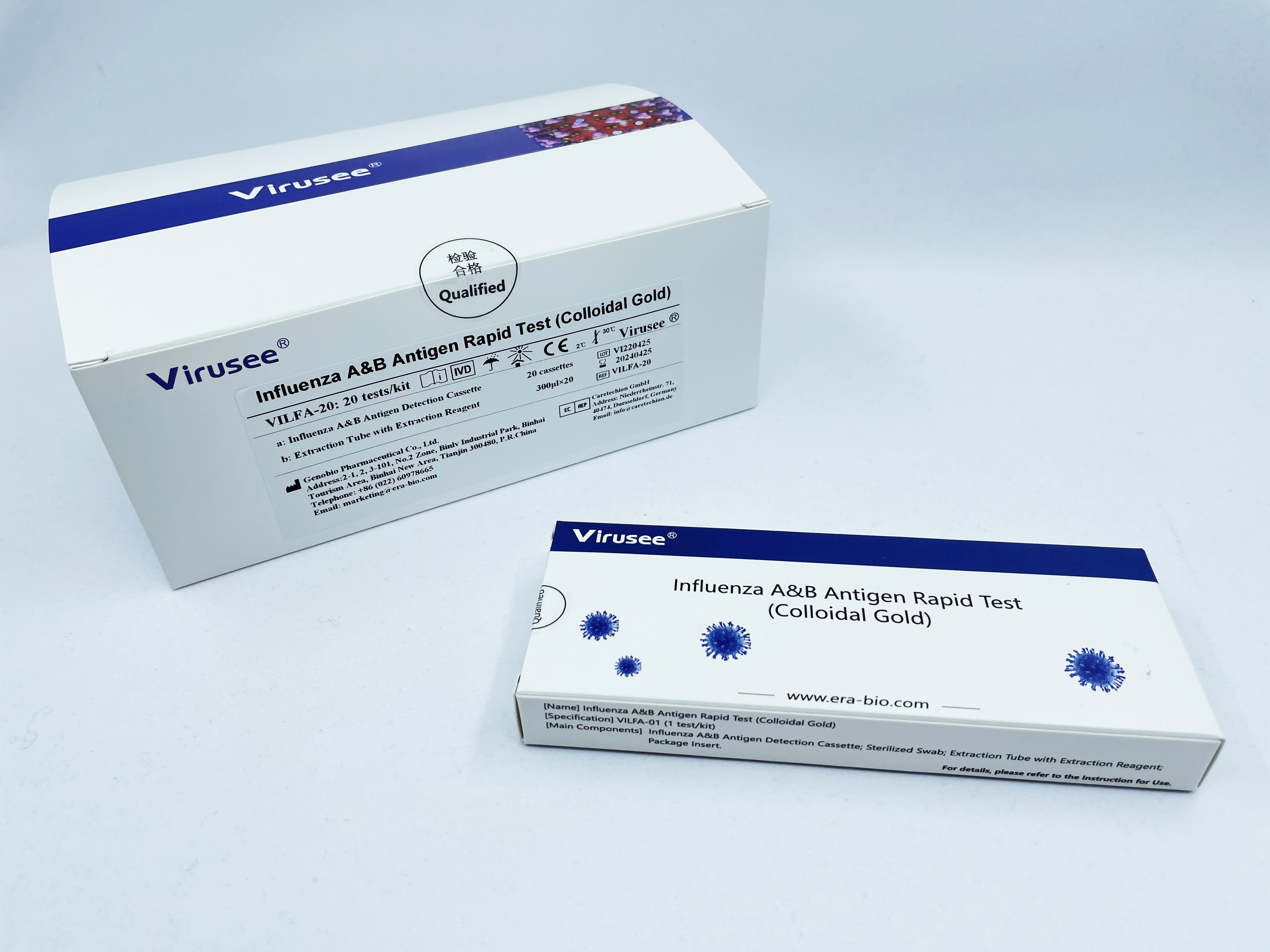
Influenza A&B Antigen Rapid Test (Colloidal Gold) is an in vitro immunochromatographic test, which is used to qualitatively detect influenza A and B virus nucleoprotein antigens in nasopharyngeal swab and oropharyngeal swab samples, and can quickly assist in the differential diagnosis of influenza A and B virus infection.
Rapid diagnostic tests for influenza A and B are of great significance for obtaining effective antiviral therapy. Rapid diagnosis of influenza not only reduces the number of days in hospital and the use of antibiotics, but also reduces hospital costs. The Influenza A&B Antigen Rapid Test (Colloidal Gold) provides a simple and rapid method for the diagnosis of influenza A and B using nasopharyngeal swab and oropharyngeal swab samples. It is easy to use and provides rapid results. The information provided during the emergency department examination helps doctors select treatment options and make decisions about whether to hospitalize or not.
Characteristics
|
Name |
Influenza A&B Antigen Rapid Test(Colloidal Gold) |
|
Method |
Colloidal Gold |
|
Sample type |
Nasopharyngeal swab, Oropharyngeal swab |
|
Specification |
1 test/kit; 20 tests/kit |
|
Detection time |
15 min |
|
Detection objects |
Influenza A and B virus nucleoprotein antigens |
|
Stability |
The K-Set is stable for 2 years at 2-30°C |
|
Low detection limit |
5×102.50TCID50/mL of Influenza A, 5×102.50TCID50/mL of Influenza B (cultured virus) |
-
Advantage
- Flexible
The sample type is optional between nasopharyngeal swab and oropharyngeal swab, convenient and meets different needing - Rapid
Obtain result within 15 min - Simple
Easy to use, without complicated operation - Economic
Product can be transported and stored at room temperature, reducing costs - Low risk
Testing swab sample, reducing the risk of sampling process
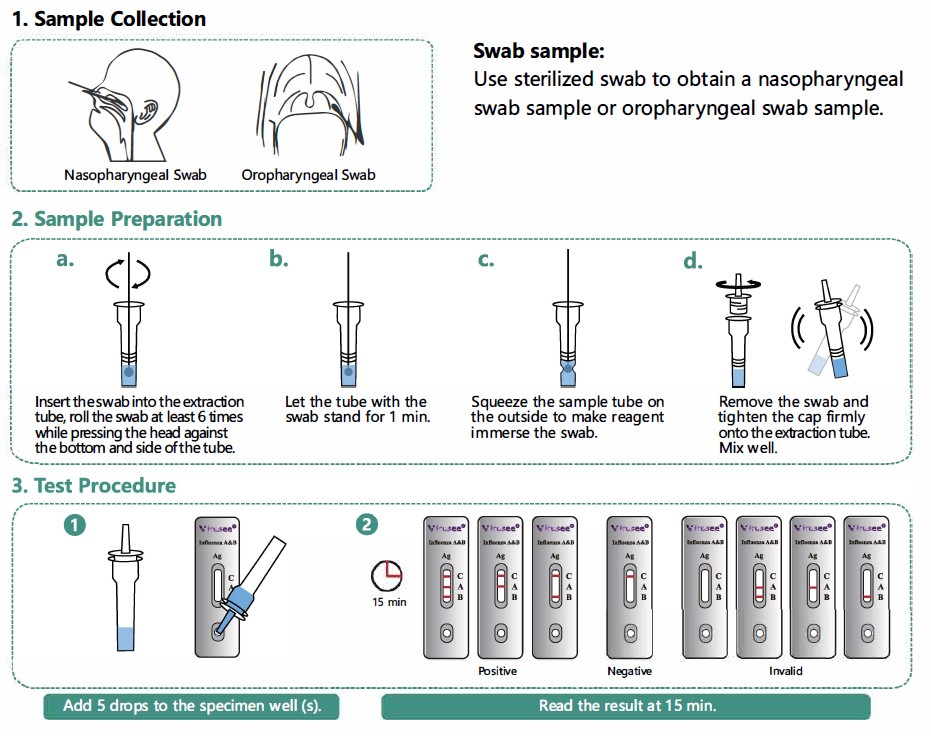
Operation
Order Information
|
Model |
Description |
|
VILFA-01 |
1 test/kit, cassette format |
|
VILFA-20 |
20 tests/kit, cassette format |
We’ve got quite possibly the most state-of-the-art production gear, experienced and qualified engineers and workers, acknowledged top quality handle systems along with a friendly expert gross sales group pre/after-sales support for China OEM Test Flu Influenza a&B Virus Antigen Diagnostic Test Kit, We’ve been extremely aware of excellent, and have the certification ISO/TS16949:2009. We are dedicated to supply you high quality solutions with acceptable price tag.
China OEM China Influenza a&B Rapid Test and Flu Test Kit, Now we have a strict and complete quality control system, which ensures that each product can meet quality requirements of customers. Besides, all of our goods have been strictly inspected before shipment.